EU MDR Clinical Evaluation for MedTech Start-Ups
How to Build Compliant Evidence with Limited Resources
“Sometimes the best way forward is simply to talk through the challenges with someone who has walked the path before; and who understands that protecting patients and building a successful business are not opposing goals, but two sides of the same coin”
EXECUTIVE SUMMARY
EU MDR Clinical Evaluation is not an administrative hurdle but a foundational process for building safe, credible, and commercially sustainable MedTech products.
Start-ups; particularly academic and clinician-led ventures; often underestimate the gap between published research and MDR-compliant clinical evidentiary expectations.
Treating Clinical Evaluation as a strategic, forward-looking activity enables earlier design alignment, clearer evidence planning, and reduced downstream regulatory risk.
Early understanding of the State-of-the-Art and existing clinical evidence allows organisations to focus clinical investigations only on genuine evidentiary gaps.
With the right mindset and disciplined planning, limited resources need not prevent start-ups from establishing a robust, MDR-ready clinical evidence base.
INTRODUCTION
For many MedTech start-ups, regulatory requirements are often seen as a hurdle; something to manage once the “real work” of innovation is complete. Under the EU Medical Device Regulation (MDR), however, this mindset is increasingly misaligned with reality. Clinical Evaluation should not be a retrospective exercise; it is a core component of product development that directly impacts design decisions, clinical strategy, and time to market.
This disconnect is particularly evident for early-stage companies emerging from academic or clinical environments. Despite strong scientific credentials and promising ideas, founders are frequently surprised to learn that a scientific paper, even one published in a leading, peer-reviewed medical journal, does not automatically meet MDR Clinical Evaluation requirements. Regulatory costs extend far beyond a single submission milestone, and the expectations for a structured, well-documented clinical evidence base are non-negotiable, regardless of company size or funding stage.
Having transitioned from academia into regulatory affairs myself, I have seen how innovative ideas can stall when MDR obligations are approached too late or treated as a documentation exercise rather than a strategic process. Yet these challenges are not insurmountable. When regulatory requirements are reframed as a strategic tool rather than a barrier, they support entry into a stable, high-trust market, protect patients, and strengthen commercial credibility.
This article explores how MedTech start-ups with limited resources can navigate MDR Clinical Evaluation effectively; adopting a mindset that integrates regulatory planning early, leverages existing clinical evidence intelligently, and turns compliance into a competitive advantage.
Build regulatory confidence from day one
INDUSTRY CONTEXT & BACKGROUND
Under the EU MDR, Clinical Evaluation has evolved from a largely retrospective documentation exercise into a strategic component of product development that is advantageous to implement early on in the development process. Many start-ups emerging from academic or clinical environments underestimate how much regulatory expectations differ from scientific publications: what counts as strong evidence in a journal may not meet MDR standards for demonstrating safety and performance.
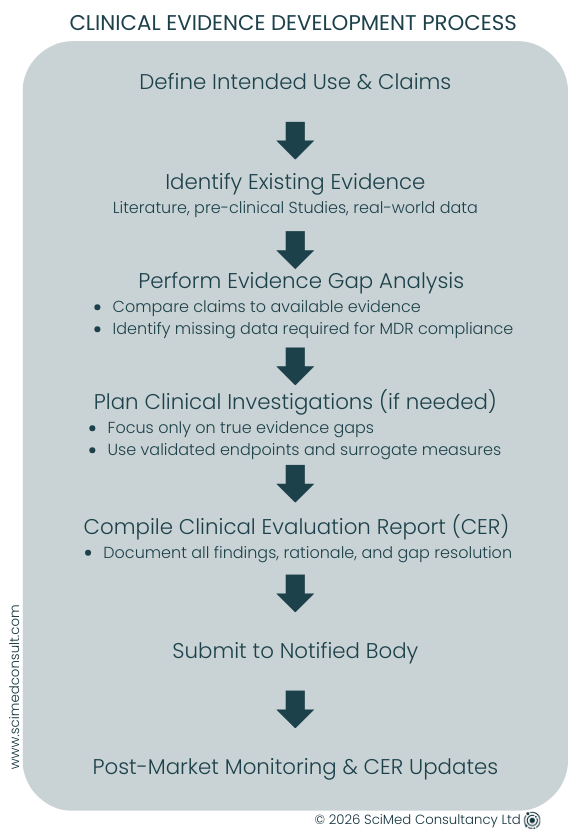
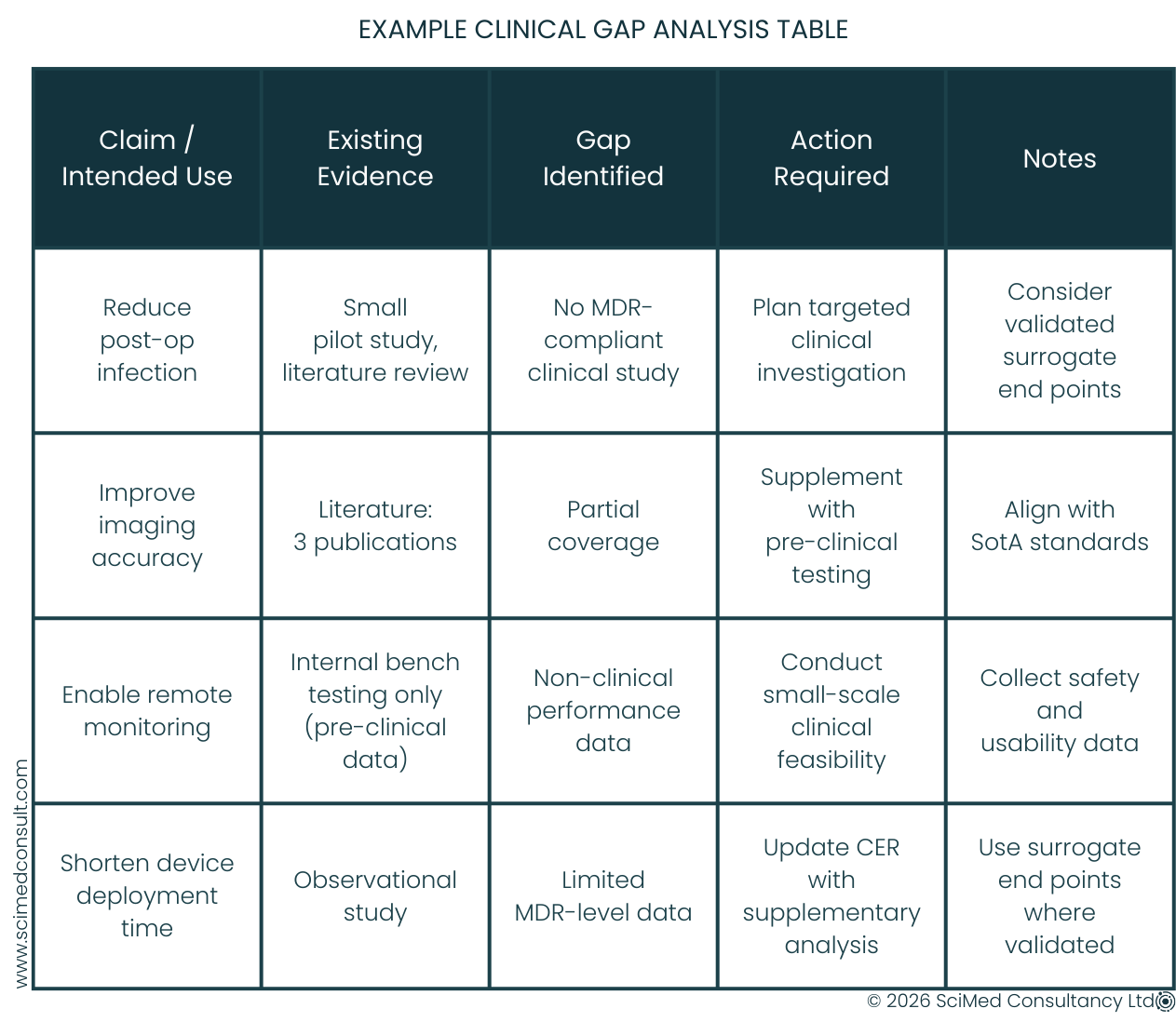
MDR now requires manufacturers to critically assess all existing clinical data, identify gaps, and plan proportionate investigations to fill those gaps. This process ensures that devices entering the market are aligned with the State-of-the-Art, claims, and intended use, while providing a foundation for predictable regulatory approval and market access.
For resource-constrained start-ups, this regulatory shift underscores the advantages of embedding Clinical Evaluation early in product development. By treating regulatory compliance as a strategic, value-adding activity rather than a post-hoc hurdle, companies can reduce risk, avoid costly rework, and turn clinical evidence planning into a competitive advantage; helping even small teams demonstrate credibility and reliability to regulators, investors, and ultimately patients.
Shift Your Perspective on Regulatory Compliance
Instead of viewing MDR as an obstacle, consider it a tool for building trust and predictability. Compliance signals to regulators, investors, and patients that your start-up is delivering safe, reliable, and high-quality devices.
Pro Tip: Lock Intended Use and Claims Earlier Than Feels Comfortable.
Embed Clinical Evaluation Early
The best way to manage limited resources is to integrate your Clinical Evaluation procedure into the design process. Planning ahead ensures that:
Pre-clinical and literature-based evidence is captured efficiently.
Clinical investigations are only performed when necessary.
Evidence generation aligns directly with claims and intended use.
Focus on Evidence, Not Publications
A high-impact journal article does not automatically meet MDR standards. The focus must be on:
Linking evidence to specific safety and performance endpoints.
Addressing State-of-the-Art benchmarks.
Documenting rationale for any gaps or surrogate endpoints.
Plan and Scope Clinical Investigations Strategically
Clinical investigations are more common under MDR than under MDD, but jumping straight into them without proper planning can create unnecessary costs and complexity. Start-ups should:
Identify evidence gaps from existing clinical data.
Scope trials to only address those gaps.
Use validated endpoints and surrogate measures where appropriate.
Integrate results directly into the Clinical Evaluation Report (CER).
Turn Compliance Into Competitive Advantage
With foresight, discipline, and early planning, even resource-constrained start-ups can establish credibility, satisfy Notified Bodies, and enter the market confidently. Regulatory compliance becomes part of your strategic toolkit, not just a cost centre.
Conclusion
Regulatory compliance in MedTech is not a barrier to innovation; it is the foundation upon which patient-centred, sustainable innovation is built. Start-ups navigating MDR Clinical Evaluation with limited resources can succeed by embracing regulatory obligations early, planning clinical evidence strategically, and treating compliance as a strategic advantage rather than a checklist.
Even the smallest ventures can carve out a credible path to market with foresight, discipline, and a commitment to quality and safety.
Like this article? Get it as a downloadable PDF.
Simply enter your contact details to access the file.
USEFUL REFERENCES
The EU MDR (Reg. (EU) 2017/745) can be found here.