Building Sustained MDR Audit Readiness
How Innovia Regained Control of Clinical Documentation and Restored Regulatory Confidence
Project Type: Clinical Evaluation Documentation Suite, Ongoing Literature Monitoring and SotA Updates
INTRODUCTION
Innovia, a long-established medical device manufacturer operating across multiple markets, faced mounting regulatory pressure following its acquisition by an investment group. When a new Head of Clinical joined the organisation in 2023, it became clear that large parts of the clinical documentation portfolio were out of compliance and that the company was insufficiently prepared for EU MDR transition audits.
To address this risk, Innovia partnered with SciMed, a specialist regulatory and clinical affairs consultancy, to remediate legacy clinical documentation, restore audit readiness, and establish predictable delivery against MDR requirements. Since the collaboration started in 2022, SciMed have supported Innovia in remediating more than 250 clinical and post-market documents; helping the business move from reactive firefighting to sustained compliance.
KEY TAKEAWAYS: How SciMed Supported Innovia’s MDR Transition
Supplemented Innovia’s remediation of more than 250 clinical and post-market documents, aligned to EU MDR expectations
Supported Innovia in achieving and maintaining an audit-ready state
Reduced notified body friction through clearer, more defensible clinical documentation
Strengthened senior management confidence through predictable delivery and reporting
THE CHALLENGE: Regulatory Risk and Limited Visibility
When Innovia began preparing for MDR transition, several challenges became apparent. There was no consolidated view of which clinical documents were due for update, limited visibility of regulatory risk across a diverse legacy portfolio, and insufficient internal capacity to remediate documents at the required pace.
Compounding this challenge was the nature of Innovia’s products. Many devices had been on the market for decades, meaning historical clinical evidence was incomplete or fragmented. While this scenario is common for legacy portfolios, it significantly increases the complexity of MDR-aligned Clinical Evaluation.
With notified body audits possible at any time, the business faced both compliance and reputational risk. Innovia needed immediate, credible support to stabilise the situation while simultaneously building internal clinical capability.
THE SOLUTION: A Pragmatic, MDR-Aligned Remediation Partnership
SciMed worked closely with Innovia’s clinical team to deliver a structured remediation programme aligned to EU MDR requirements, including Clinical Evaluation Reports, PMCF-related documentation, and supporting clinical evidence outputs.
Rather than applying a rigid or academic approach, SciMed focused on striking the right balance between good scientific practice and pragmatic delivery, particularly important for legacy devices where ideal data sets were not available. Documentation was developed with a strong emphasis on clarity, rationale, and defensibility, ensuring notified body reviewers could easily follow the clinical logic.
SciMed also supported improvements in working practices, including document templates, review workflows, and reporting rhythms that aligned with senior management oversight. This allowed Innovia’s leadership team to clearly track progress and maintain confidence in delivery.
A short, confidential conversation to understand your MDR challenges and explore practical next steps.
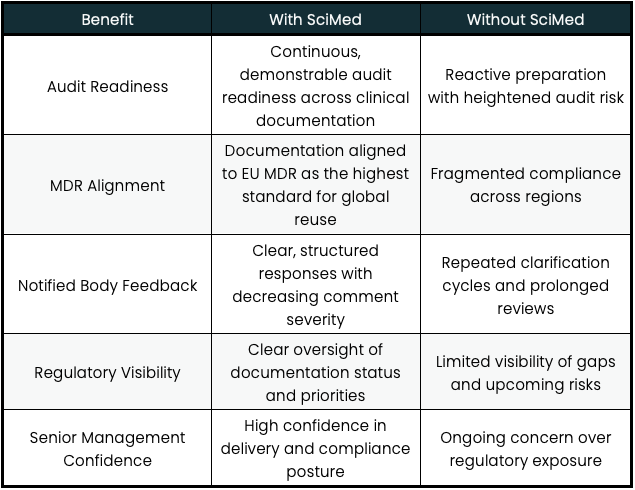
THE RESULTS: From Firefighting to Audit-Readiness
Over the course of the engagement, SciMed helped Innovia remediate hundreds of documents, consistently meeting internal targets. Most importantly, the organisation moved from a position of being “never audit ready” to maintaining a continuous state of readiness.
Feedback from notified bodies became more manageable over time, with a clear reduction in the severity and volume of comments on clinical documentation. Internally, confidence increased across the clinical team and senior leadership, supported by predictable delivery and improved regulatory visibility.
CONCLUSIONS: Building Sustained Compliance, Not Just Documents
Innovia’s experience demonstrates the value of a long-term, collaborative regulatory partnership. By combining pragmatic clinical expertise with structured delivery, SciMed helped Innovia regain control of its clinical documentation portfolio and embed sustainable compliance practices.
For manufacturers facing MDR transition with complex legacy devices, Innovia’s journey shows that audit readiness is achievable with the right balance of expertise, partnership, and process.